Abstract
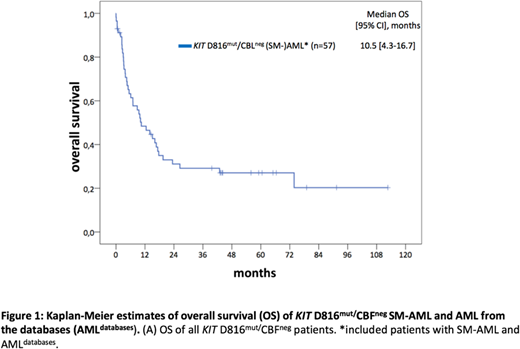
Systemic mastocytosis with an associated hematologic neoplasm (SM-AHN) is the most common subtype of advanced SM (advSM), diagnosed in up to 80% of patients. The AHN is most frequently diagnosed as a myeloid neoplasm, e.g., SM-MDS/MPNu or SM-CMML. Acquired mutations in KIT (usually KIT D816, KIT D816mut) are detectable in >90% of patients. The basis for the SM-AHN phenotype is usually the multi-lineage involvement, e.g. monocytes, eosinophils and other non-mast cell lineages, of KIT mutations. Core binding factor (CBF) positive AML (CBFpos AML) represents a distinct subtype and is identified in 5-8% of all AMLs. KIT mutations, most frequently KITD816mut, are detectable in up to 45% of CBFpos AML patients and are associated with an adverse prognosis. There is, however, only little information on KIT D816mut/CBFneg AML. We therefore evaluated a) clinical and molecular characteristics, b) response to treatment and, c) survival and prognostic factors in 40 KIT D816mut/CBFneg patients with histologically proven SM and associated AML (SM-AML), collected at 4 centers of the European Competence Network on Mastocytosis (ECNM). Molecular analyses (n=32) revealed at least one additional somatic mutation (median, n=3) apart from KIT D816, most frequently SRSF2 (n=12, 38%), RUNX1 (n=11, 34%), TET2 (n=11, 34%), ASXL1 (n=10, 31%), or NPM1 (n=7, 22%). At least one mutation in SRSF2, ASXL1 or, RUNX1 (S/A/Rpos) was identified in 21/32 (66%) patients. At diagnosis of SM-AML 21/40 (52%) patients had an aberrant karyotype. Secondary AML evolved in 29/40 (73%) patients from SM ± associated myeloid neoplasm and longitudinal molecular analyses revealed acquisition of new somatic mutations (TP53, n=2; NPM1, n=1; RUNX1, n=1, ASXL1, n=1; BCOR, n=1; IDH1/2, n=1) and/or karyotype evolution in 15/16 (94%) patients at the time of SM-AML. Thirty-one of 40 (78%) patients were treated with intensive chemotherapy (ICT) with a complete response (CR) rate of 40%. Allogeneic stem cell transplantation (SCT) was performed in 12/40 (30%) patients with durable CR in 6/12 (50%) patients. S/A/Rpos and/or the presence of a poor-risk karyotype were adverse predictive markers for response to treatment. To further investigate whether KITD816mut/CBFneg AMLdefines a distinct AML subtype associated with SM, two independent AML databases (AMLdatabases) were retrospectively screened and 69 KIT D816mut/CBFneg AML patients identified. The comparison between KIT D816mut/CBFneg SM-AML from ECNM (n=40) centers with KIT D816mut/CBFneg AMLdatabases(n=69) revealed remarkable similarities: a) a high KIT D816 variant allele frequency (VAF) (median 34% vs. 29%), b) with the exception of SRSF2 (38 vs. 18%), a highly similar mutation landscape, rather comparable to that of advSM (Jawhar et al., Blood 2017) than to that of de novo AML, c) in contrast to de novo AML, a low frequency of FLT3 mutations (3 vs. 7%), and d) a high frequency of an aberrant karyotype (52 vs. 42%). The median overall survival (OS) of 40 KIT D816mut/CBFneg SM-AML and 17 evaluable KIT D816mut/CBFneg AMLdatabases was 5.4 (95% confidence interval, CI [1.7-9.1]) and 26.4 (95% CI [0-61.0]) (P=0.015) months, respectively (Figure 1). However, if only the patients with ICT ± allogeneic SCT were compared, median OS between the two groupswas not different (16.7 vs. 26.4 months, P=0.4). In multivariate analyses, S/A/Rpos and a poor-risk karyotype remained the only independent poor-risk factors with regard to OS. These results were independent of treatment modalities. We conclude that KIT D816mut/CBFneg AML is a new poor-risk subtype associated with SM (SM-AML). The remarkable clinical, genetic and prognostic similarities between SM-AML and AMLdatabases suggest that a significant proportion of the AMLdatabases patients may in fact have SM-AML. We therefore strongly recommend to determine serum tryptase and KIT D816 mutation status in all AML patients, and to perform bone marrow histology in KIT D816mut patients. These simple diagnostic measures would allow reclassification to SM-AML and inclusion of KIT inhibitors in established treatment modalities of AML.
Meggendorfer:MLL Munich Leukemia Laboratory: Employment. Haferlach:MLL Munich Leukemia Laboratory: Employment, Equity Ownership. Döhner:Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; AROG Pharmaceuticals: Research Funding; Amgen: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Research Funding; Astellas: Consultancy, Honoraria; Celator: Consultancy, Honoraria; Bristol Myers Squibb: Research Funding; Celator: Consultancy, Honoraria; Sunesis: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Astex Pharmaceuticals: Consultancy, Honoraria; AROG Pharmaceuticals: Research Funding; Astellas: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Astex Pharmaceuticals: Consultancy, Honoraria; Agios: Consultancy, Honoraria; Bristol Myers Squibb: Research Funding; Jazz: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Pfizer: Research Funding; Pfizer: Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Seattle Genetics: Consultancy, Honoraria; Sunesis: Consultancy, Honoraria, Research Funding. Sperr:Novartis: Honoraria; Pfizer: Honoraria; Daiichi Sankyo: Honoraria. Valent:Incyte: Honoraria; Pfizer: Honoraria; Novartis: Honoraria. Reiter:Incyte: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal